I am looking for information as
Researcher

Industry

Patient

Frequently Asked Questions
All you need to know about us
What is BBMRI-ERIC?
BBMRI-ERIC is a distributed research infrastructure of biobanks and biomolecular resources. For its Member States, it provides expertise and services on a non-economic basis and facilitates access to collections of partner biobanks and biomolecular resources. BBMRI-ERIC is established for an unlimited period of time.
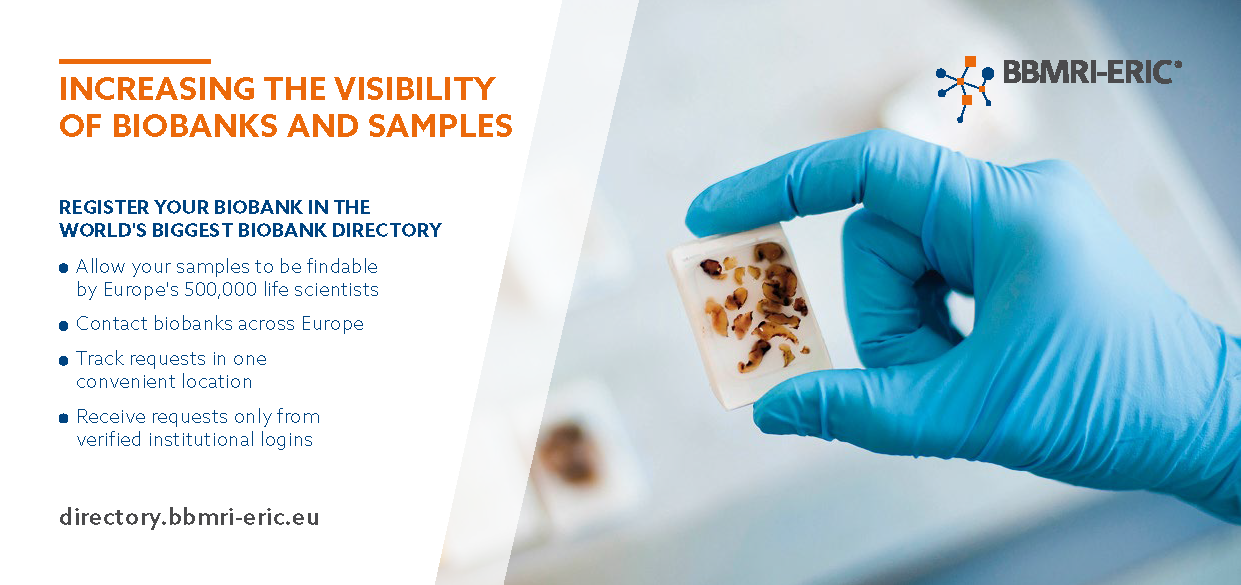
Why is the work BBMRI-ERIC does important?
BBMRI-ERIC works towards connecting biobanks and researchers in Europe in order to facilitate the use of samples/data collected in Europe for the benefit of human health. We help researchers to find the samples and data they need for their research through better utilisation of quality-defined biobanks and their samples/data in an ethically and legally compliant manner. We help biobanks with their visibility, quality development and usability and advise them onethical and legal questions such as the European regulation framework. We help European citizens by taking on the responsibility of paving the way from high-quality research to discovery.
What can BBMRI-ERIC help with?
We facilitate collaboration in a very competitive field of biomedical science. While the funding sources of biomedical science and its supportive elements (e.g., biobanks) are scarce and the competition is intense, BBMRI-ERIC builds services for the common needs of the research community. BBMRI-ERIC facilitates collaboration on many levels (e.g., IT, ELSI and Quality) for the benefit of the whole research community in Europe.
What is the main purpose of BBMRI-ERIC?
According to its Statutes (Article 3,1),
BBMRI-ERIC shall establish, operate and develop a pan-European distributed research infrastructure of Biobanks and Biomolecular Resources in order to facilitate the access to resources as well as facilities and support high-quality biomolecular and medical research. BBMRI-ERIC shall implement its work programme as adopted by the Assembly of Members.
What does BBMRI-ERIC stand for?
Biobanking and BioMolecular resources Research Infrastructure
European Research Infrastructure Consortium
What are the BBMRI-ERIC Statutes?
The Statutes of BBMRI-ERIC specify the general and financial provisions as well as governance and management. They entered into force on 3 December 2013.
What is the BBMRI-ERIC Partner Charter?
The BBMRI-ERIC Partner Charter was developed during the BBMRI Preparatory Phase (BBMRI-PP) and approved by the BBMRI-PP Steering Committee for inclusion into the Business Plan 2012. Consequently, the Partner Charter became part of the official dossier for countries to later establish BBMRI-ERIC. Ultimately, the Assembly of Members approved the Partner Charter unchanged during its 2nd Session in April 2014.
For biobanks, the Partner Charter defines cornerstones to participate as partners in the wider BBMRI-ERIC network or within BBMRI-ERI- led research projects. It should be signed between the National Node (its legal representative) and the Partner (its legal representative). This can be done by including or referring to the Partner Charter in a National Consortium Agreement (an agreement potentially established at National level for all participating biobanks – see also our standardisation page), as part of an application or through individual signatures. The appointed National Coordinator is responsible for this implementation and will report to the HQ.
The Partner Charter does neither replace nor change national or European legislation, rather, it is a statement of intention. A cornerstone of Quality Management is continuous monitoring and evaluation. This also includes the implementation of regular audit sessions. BBMRI-ERIC’s role is to oversee that such audits are done, either as self-assessments, internal audits or external audits (not to be confused with a Certification Audit by a national body).
What is an ERIC?
An ERIC is a specific legal form designed to facilitate the joint establishment and operation of research infrastructures of European interest.
What is a research infrastructure?
The European Commission specifies:
Research infrastructures (RIs) play an increasingly important role in the advancement of knowledge and technology. They are a key instrument in bringing together a wide diversity of stakeholders to look for solutions to many of the problems society is facing today. RIs offer unique research services to users from different countries, attract young people to science, and help to shape scientific communities.
New knowledge and, by implication, innovation, can only emerge from high-quality and accessible RIs: for example, radiation sources, data banks in genomics, observatories for environmental sciences, systems of imaging or clean rooms for the study and development of new materials or nano-electronics are at the core of research and innovation processes. Moreover, RIs help to create a new research environment in which all researchers – whether working in the context of their home institutions or in national or multinational scientific initiatives – have shared access to unique or distributed scientific facilities (including data, instruments, computing and communications), regardless of their type and location in the world. RIs are therefore at the centre of the knowledge triangle of research, education and innovation, producing knowledge through research, diffusing it through education, and applying it through innovation.
Further information see http://ec.europa.eu/research/infrastructures/index_en.cfm
Watch our informational animation on European Research Infrastructures here.
Is BBMRI-ERIC an EU project?
No. BBMRI-ERIC is a European organisation established under the ERIC legal framework, funded by yearly membership fees paid by the Member States.
Who are the members of BBMRI-ERIC?
Members of BBMRI-ERIC are Member States, third countries as well as intergovernmental organisations. A full list of current Members and Observers can be found here.
How do I become a Member or Observer of BBMRI-ERIC?
Only countries and intergovernmental organisations can become Members or Observers of BBMRI-ERIC. A formal request signed by a competent authority (e.g., Ministry of Research) needs to be submitted to the Director General of BBMRI-ERIC and the Assembly of Members decides in its next session on the admission. Observers participate in the deliberations of BBMRI-ERIC but due to their reduced contributions have no voting powers.
For further details please contact Head of Public Affairs Jana Pavlic-Zupanc.
If my country is already a member or observer of BBMRI-ERIC, what can I do?
If your biobank is located in a Member country of BBMRI-ERIC and you would like to get involved and your biobank to be listed in the BBMRI-ERIC Directory, contact the respective National Node Director in your country. Contact details can be found here.
What is a Common Service?
A Common Service is a key facility providing expertise, services and tools relevant for the pursuance of the tasks and activities of BBMRI-ERIC.
What is the Common Service IT?
The Common Service IT aims to implement core IT services, such as the Directory, the Sample/Data Locator and Negotiator as well as data harmonisation tools and reference tools for biobanks.
What are the services of BBMRI-ERIC in general?
The Services of BBMRI-ERIC cover a wide range of user needs providing the following services, tools and advice:
- IT (e.g., Directory for biobanks and their users)
- ELSI (e.g., FAQs on the General Data Protection Regulation)
- Quality (e.g., consultancy to improve quality systems in biobanks)
- Biobanking Development
What are the Services of BBMRI-ERIC for ELSI?
ELSI Services & Research offers support on ethical, legal and societal issues related to biobanking activities, among them practical interpretation of new legislation monitoring ethical and legal frameworks in development, developing legal tools to support biobankers in their daily work, informing about publications, research results, surveys and relevant meetings, providing an ethics check for ELSI in compliance for research proposals, organising experience sharing. Its services are primarily intended for users located in Member Countries of BBMRI-ERIC.

What are the Services of BBMRI-ERIC for IT?
The Services for IT support both biobanks and the users of the biobanks. Particular focus is to improve the visibility and findability of biobanks, biobanked samples and associated data. The Directory of BBMRI-ERIC places the biobanks and sample collections in the map supports findability, while other tools and services for the IT enables and improves the effective communication between the biobanks and the requester’s of biobanked samples and data. Its services are primarily intended for users located in Member Countries of BBMRI-ERIC.
What are the Services of BBMRI-ERIC for Quality?
BBMRI-ERIC offers support with quality management related to biobanking activities: quality management consultancy programmes for guidelines and standards, quality management monitoring and audit programmes, quality management training and education formats, quality management documentation and assessment. Its services are primarily intended for users located in Member Countries of BBMRI-ERIC.
What is an Expert Centre?
“BBMRI-ERIC associated Expert Centres/Trusted Partners” build the bridge between academic and applied research in a public-private partnership model; by integrating pre-competitive research and development endeavours by providing not only access to biological samples and medical data but access also to the broad spectrum of medical and scientific expertise related to the samples, data, and their analysis. BBMRI-ERIC Expert Centres are closely linked to the vision and mission strategy of BBMRI-ERIC.
How can my entity be approved as an Expert Centre/Trusted Partner?
In 2015, BBRMI-ERIC developed an approval process for BBMRI-ERIC Expert Centres/Trusted Partners, as referred to in the ‘Business Plan’ (version 21.1 of 3 December 2012) and the ‘Work Programme 2015’. The approval process is described in the specification document BBMRI-ERIC Expert Centres/Trusted Partners_V1
What is a National Node?
According to the Statutes (Chapter 1, Article 1,6), a
‘National Node’ means an entity, not necessarily with legal capacity, designated by a Member State, that coordinates the national biobanks and biomolecular resources, and links its activities with the pan-European activities of BBMRI-ERIC.
What is an Organisational Node?
According to the Statutes (Chapter 1, Article 1,8), an
‘Organisational Node’ means an entity, not necessarily with legal capacity, designated by an intergovernmental organisation that coordinates the biobank(s) and biomolecular resources of the organisation, and links its activities with those of the pan-European infrastructure, BBMRI-ERIC.
What is a sample?
In order to avoid any conflicting definitions, BBMRI-ERIC is waiting to communicate the ISO/TC 276 committee work on terminology as soon as it is available. BBMRI-ERIC actively participates to and supports the ISO committee work. Currently, a ‘sample’ has been defined as:
- A biological specimen from the human body including e.g. tissue, blood, blood components, cell lines and biopsies. (by MIABIS)
- A biological specimen from the human body including e.g. tissue, blood, blood components, cell lines and biopsies … / portions of biospecimens distributed to researchers (by P3G)
- Portions of biospecimens distributed to researchers. (by IARC)
- A single unit containing material derived from one specimen. (by ISBER)
- One or more parts taken from a primary sample. (by DIN EN ISO 15189:2014)
- A primary sample specimen – discrete portion of a body fluid, breath, hair or tissue taken for examination, study or analysis of one or more quantities or properties assumed to apply for the whole (by CEN/TS 16835-1-3:2015, CEN/TS 16945:2016)
What are BRIF and CoBRA? Why are they useful for refering to bioresources?
The BRIF (Bioresource Research Impact Factor) initiative is building a framework that enables set up indicators for the use of bioresources* and rewarding mechanisms. Generated as part of the BRIF initiative, the CoBRA (Citing Of Bioresources in Research Articles) guideline provides guidance for citing bioresources in academic literature: it specifies where and how to cite bioresources at each section of a research article. The guideline is intended for any researcher or professional reporting on a research work using bioresources or referring to bioresources in a scientific journal article.
To use the CoBRA checklist when writing a scientific article download the guideline at: http://www.equator-network.org/reporting-guidelines/cobra/
You may also be interested in
National Nodes & contact points for local biobanks
![]() Find our National Nodes
Find our National Nodes
and contact points for local biobanks.
Merken
Merken
Merken
... read moreBBMRI.QM Academy E-Learning
Live educational webinars and webinars on demand (recordings) on Pre-Analytical Phases of Biobanking.
... read more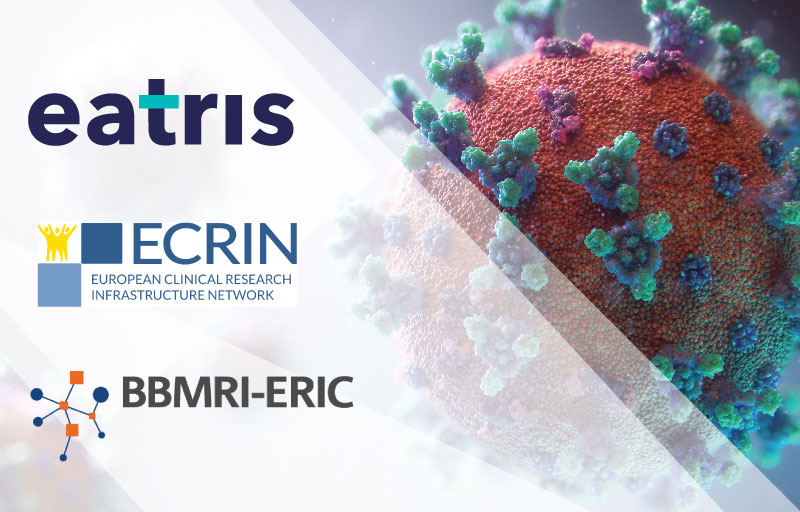
COVID-19: European Medical Research Infrastructures are Part of the Global Response
Together with EATRIS and ECRIN we just published our top 5 recommendations to accelerate #COVID19 research...... read more

BBMRI-ERIC receives two new grants for coronavirus initiatives
With BY-COVID and ISIDORe, BBMRI-ERIC will contribute to European preparedness for current and future pandemics... read more




